Medical device premarket approval and postmarket inspections.
Fda inspection checklist medical device.
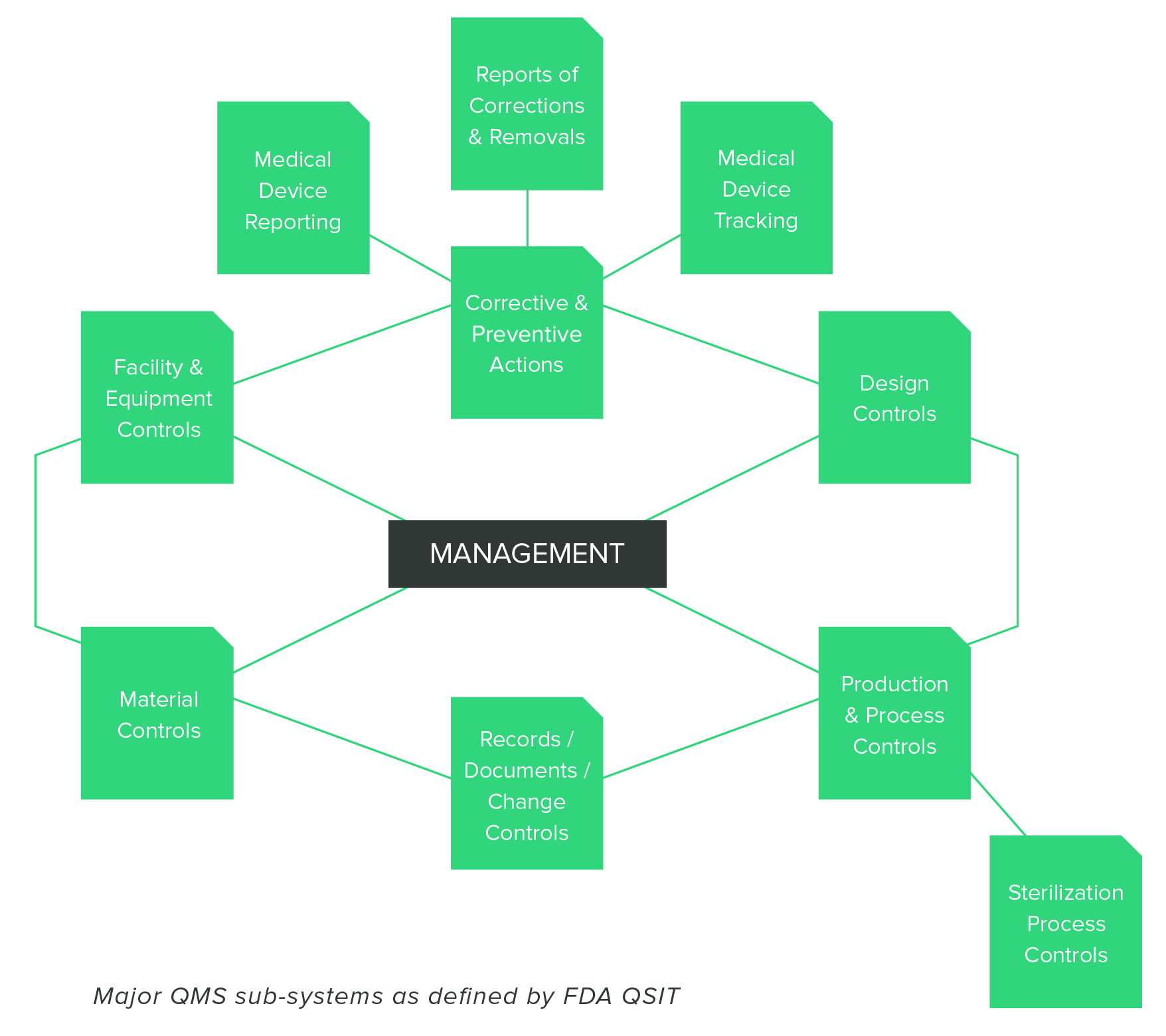
As medical device manufacturers you can expect to be inspected.
The food and drug administration fda conducts inspections and assessments of regulated facilities to determine a firm s compliance with applicable laws and regulations such as the food drug.
Have a good qms in place and keep it up to date as well as keeping up with your management reviews and internal inspections.
This document does not bind fda and does not confer any rights privileges benifits or.
Fda audit checklist when fda calls to schedule a site visit obtain the following information.
During an inspection ora investigators may observe conditions they.
Refuse to accept policy for 510 k s describes the criteria fda intends to use in assessing a 510 k submission for review.
Guide to inspections of.
Fda medical device inspections fda small business regulatory education for industry redi silver spring maryland september 30 2015 marc neubauer.
Understand what an fda inspection is why it is necessary and how you can prepare for one.
Fda s office of regulatory affairs ora is the lead office for all field activities including inspections and enforcement.
A checklist for responding to fda 483 observations and warning letters.
R compliance program guidance manual for inspection of medical device manufacturers cp 7382 845.
Checklist to be done prior to inspection if possible.
Guide to inspections of medical device manufacturers.
Step 1 gather and review study documentation detailed list follows.
Preparation checklist 2 fda inspection food manufacturing 3 fda audit pharmaceutical gmp checklist 4 fda gmp cosmetics audit checklist 5 gmp compliance checklist.
Device studies all versions.